Background
Clever-1 (common lymphatic endothelial and vascular endothelial receptor-1) constitutes a novel macrophage checkpoint. The receptor contributes to immune tolerance by antigen clearing and T cell inhibition. In multiple models, tumor growth is significantly reduced when Clever-1 function is inhibited. Expression of Clever-1 on bone marrow (BM) cells is a prognostic factor for poor outcome in acute myeloid leukemia (AML) and suppression of Clever-1 signaling inhibits leukemia cell growth.
Bexmarilimab (BEX), a humanized IgG4 monoclonal antibody, binds Clever-1 resulting in increased antigen presentation, secretion of proinflammatory cytokines and activation of T cells.
Clever-1 is highly expressed on leukemic blasts and monocytes of AML and myelodysplastic syndrome (MDS) patients. Treatment of AML bone marrow cells with BEX alone or in combination with azacitidine/venetoclax results in enhanced antigen presentation capacity and increased activation markers on effector T cells with synergistic effect on cell death. Also, BEX treatment alone lowers blast metabolic fitness via interference with mitochondrial oxidation.
The ongoing BEXMAB clinical study investigates safety and efficacy of BEX combined with standard of care (SoC) in patients with myeloid malignancies (NCT05428969).
Method
This is a Ph1/2 study, investigating BEX combined with azacitidine (doublet) in patients with higher risk MDS frontline or hypomethylating agent (HMA)-failed, and relapsed/refractory (r/r) AML patients.
BEX is dosed Q1W in 28-day cycles until disease progression or intolerable toxicity. SoC azacitidine is administered as per label. During Ph1, BEX doses were escalated from 1.0 mg/kg to 6.0 mg/kg (Bayesian optimal interval [BOIN] design). BM was analyzed at the end of Cycle 1 and 3 followed by every three cycles.
Ph2 will start with a dose optimization per malignancy followed by a dose expansion for efficacy evaluation (2-Stage design).
Results
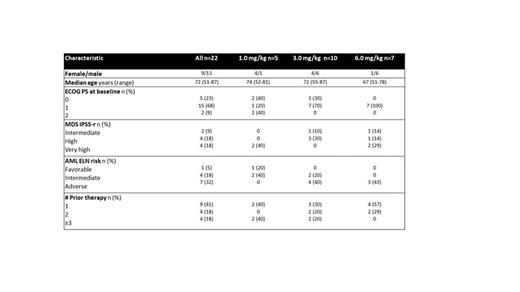
Here we report data from the dose finding Ph1 of the doublet. As of 25 July 2023, 22 patients have been enrolled in the doublet (MDS n=5; MDS HMA failed n=5; r/r AML n=12). Baseline characteristics are shown in Table 1. 8/10 MDS/MDS HMA-failed patients were of high to very high risk based on rIPSS; of the r/r AML 7/12 patients had adverse risk based on ELN 2017. The median number of prior therapies for the MDS HMA-failed patients was 1 (range 1-2). The median number of prior therapies for the r/r AML patients was 2 (range 1-4) with all patients having failed prior HMA-containing therapies and 7/12 having received prior HMA plus venetoclax.
The majority of observed adverse events (AE) were Grade 1-2 and no dose limiting toxicities have been reported. As of 25 July 2023, a total of 10 BEX related AEs have been reported (8% of all treatment emergent AEs) with four of these events of ≥ Grade 3, two being immune related (capillary leak syndrome and hemophagocytic lymphohistiocytosis).
Sustained target engagement of soluble Clever-1 of up to 75% compared to baseline was seen at all tested dose levels in patient blood. Activity of BEX in patient BM was demonstrated using receptor binding assays with BEX treatment resulting in decreased blast Clever-1 expression at all dose levels of the doublet cohort. Increased immune activation as measured using HLA expression and T/NK cell numbers in patient BM was observed after BEX treatment across indications. Up to 2-3-fold increase of CD8 T and NK cells in the BM of BEX treated patients was observed.
Preliminary efficacy shows objective responses in 8/15 evaluable patients across all tested BEX doses. Four were observed in patients with prior HMA-failure and four patients stayed on treatment > 6 months. Clinical activity for frontline MDS was seen as one complete remission (CR), marrow CR (mCR) and hematologic improvement for platelets (HI-P); for MDS with HMA failure as one CR, mCR and partial remission (PR); for r/r AML as two incomplete CRs (CRi). Based on accumulating data, responses are observed between end Cycle 1 and end Cycle 4 for all BEX doses.
Conclusion
Combining BEX plus azacitidine is well-tolerated and shows efficacy across indications. Additional clinical and pharmacodynamic data of the completed Ph1 of the study will be presented during the meeting. Ph2 of BEX plus azacitidine will open in the 2 nd half of 2023 in HMA-failed r/r AML and/or higher risk MDS patients.
Disclosures
Kontro:BMS: Speakers Bureau; Faron Pharmaceuticals: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy. Stein:Amgen: Speakers Bureau. Pyörälä:Faron Pharmaceuticals: Consultancy. Rimpiläinen:Faron Pharmaceuticals: Consultancy. Siitonen:Faron Pharmaceuticals: Consultancy. Hollmén:Faron Pharmaceuticals: Current Employment. Fjaellskog:Faron Pharmaceuticals: Current Employment. Pawlitzky:Faron Pharmaceuticals: Current Employment. Zeidan:BioCryst: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Shattuck Labs: Research Funding; ALX Oncology: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Astex: Research Funding; Geron: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Agios: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Mendus: Consultancy, Honoraria. Daver:ImmunoGen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Syndax: Consultancy; Novimmune: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Agios: Consultancy; FATE: Research Funding; Hanmi: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Trovagene: Research Funding; Shattuck Labs: Consultancy; Celgene: Consultancy; Servier: Consultancy, Research Funding; Novartis: Consultancy; Gilead: Consultancy, Research Funding; Jazz: Consultancy; Glycomimetics: Research Funding; AROG: Consultancy; Pfizer: Consultancy, Research Funding; Kite, a Gilead company: Consultancy, Research Funding; Kronos Bio: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal